MAGNETICS
MAGNETICS



Figures:1,2,3 respectively
(1) Intrinsic diamagnetism effect
Most of the scale causing solids in fluid systems are
diamagnetic. Being diamagnetic, their nature is such that they will be
repelled by magnetic field. the most common such substances are calcium
carbonate (calcite), calcium sulphate, barium sulphate, sodium chloride,
magnesium sulphate, paraffin, oil waxes and greases.
When these substances are subjected
to a magnetic field they are polarized and take on the polarity of the
magnetic feld itself. This is called induced polarization.This polarization
is such that the magnetic field of the magnet induces a similar polarity
in the diamagnetic substances. Thus as likes repel, the diamagnetic molecule
is repelled by the magnetic field and by the other polarized diamagnetic
molecules. This characterisatic of all diamagnetic substances is due to
the fact that the diamagnetic molecule has no permanent magnetic moment
of its own On the other hand paramagnetic substances are those sustances
which have their magnetic moment and are attracted by the external magnetic
field..
Magnetic susceptibility is a value
which describes if a substance is diamagnetic or paramagnetic. If the magnetic
susceptibility of a molecule is negative the substance is diamagnetic,
if positive then it is negative.
(2) Agitation/Turbulence effects
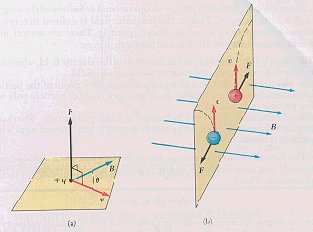
Magnetic force on a charged particle moving in a magnetic
field is
F = qv x B
Where F is the magnetic force on the charged particle
v
is velocity of the particle and B is the external magnetic field.
When v is at an angle to B, the magnetic force is perpendicular
to both v and B. In the presence of a magnetic field, the moving charged
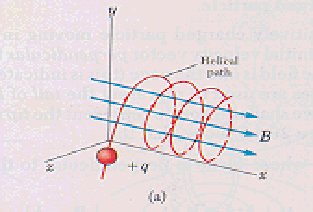
particles are deflected as indicated by the dotted lines in Figure2. leading
to helix motion as shown in Figure3. This continued action leads to turbulence,
leading to scale removal.
Effect of Magnetic field on Calcite scaling.
Study carried out by Dr.M.T.Pandya of Jai Hind College, Bombay., states that, when water molecules with dipole moment pass through magnetic fields, the polarized water molecule increases carbonic acid content due to interaction between H+,OH- and dissoved CO2. Sequence of these reactions leads to formation of Ca(HCO3)2 calcium bicarbonate resulting in increase in solubility of calcium ion. The formation of calcium bicarbonates now initiates a reversible reaction.
Ca(HCO3)2 = CaCO3 + CO2 + H2O
CaCO3 formed is in colloidal state and hence remains in suspension.
Bicarbonate formed due to interaction of polarized water
molecule and CO2 (dissolved carbondioxide)
interacts with existing scale
(CaCO3) and
converts, i.e., to Ca(HCO3)2.
Thus a series of reactions take place in short time looosening CaCO3
scales
from pipelines. Scales formed due to colloidal CaCO3
are
soft and can be easily removed.
Effect of Magnetic field on Silica scaling.
There has been little research and testing done on the
effect of magnetic field on silica scaling. Research carried out by Kevin
Brown of the University of Auckland, NewZealand, found an increase of silica
scaling by using some magnetic field creating devices.
While Hydrodynamics Corp, P.O.Box
667, Bogalusa, LA 70427, United States., which make MHD units (Magneto-HydroDynamics)
claims that there magnetic field creating devices lead silica scale build
up into such a condition that it could be washed away easily.
Reaction with other compounds could change the electronic configuration
of the molecule
Resulting in paramagnetic effect.